Determine the formulas for these ionic compounds
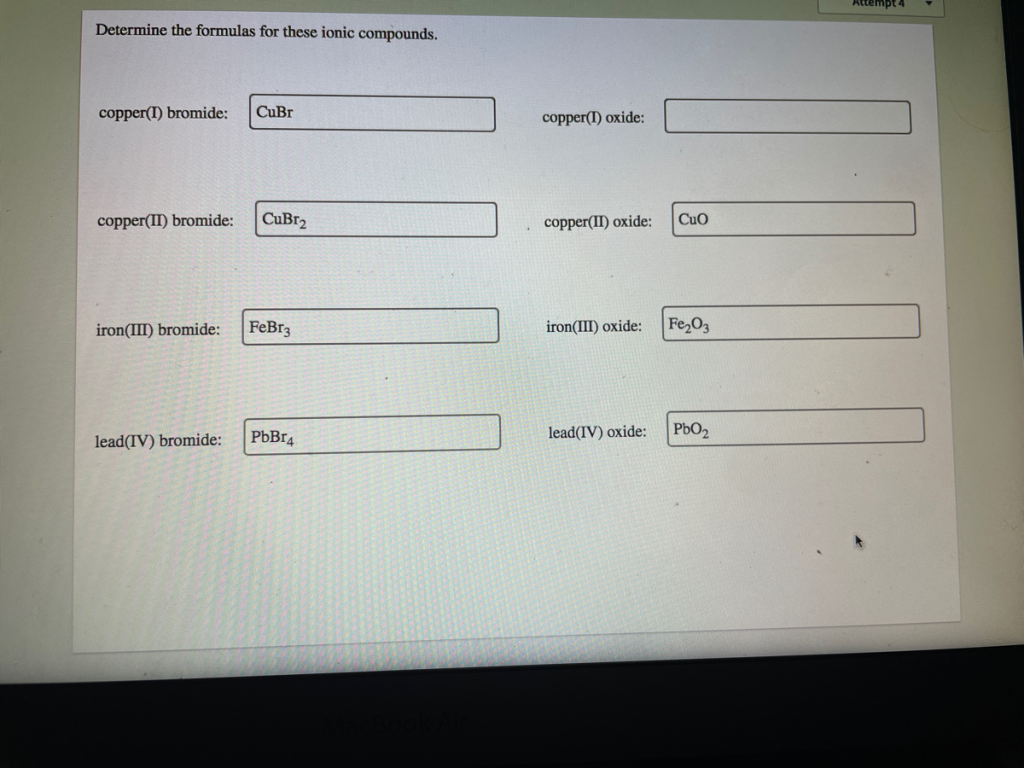
The Correct Answer and Explanation is :
To determine the formulas for ionic compounds, follow these steps:
- Identify the Cation and Its Charge: The cation is the positively charged ion, typically a metal. For example, sodium (Na) forms a cation with a +1 charge, denoted as Na⁺.
- Identify the Anion and Its Charge: The anion is the negatively charged ion, usually a non-metal or a polyatomic ion. For instance, chloride (Cl) has a -1 charge, represented as Cl⁻.
- Balance the Total Positive and Negative Charges: The total positive charge from cations must equal the total negative charge from anions to form a neutral compound. If the charges are not equal, adjust the ratio of ions accordingly.
- Write the Formula: Combine the cation and anion, using subscripts to indicate the number of each ion needed to balance the charges. The cation is written first, followed by the anion. Subscripts of ‘1’ are omitted.
Examples:
- Sodium Chloride (NaCl):
- Cation: Sodium (Na⁺)
- Anion: Chloride (Cl⁻)
- Charges: +1 (Na⁺) and -1 (Cl⁻)
- Formula: NaCl (1:1 ratio balances the charges)
- Calcium Chloride (CaCl₂):
- Cation: Calcium (Ca²⁺)
- Anion: Chloride (Cl⁻)
- Charges: +2 (Ca²⁺) and -1 (Cl⁻)
- Balancing Charges: Two chloride ions are needed to balance one calcium ion.
- Formula: CaCl₂
- Aluminum Sulfate (Al₂(SO₄)₃):
- Cation: Aluminum (Al³⁺)
- Anion: Sulfate (SO₄²⁻)
- Charges: +3 (Al³⁺) and -2 (SO₄²⁻)
- Balancing Charges: Two aluminum ions (total +6) balance with three sulfate ions (total -6).
- Formula: Al₂(SO₄)₃
Key Points:
- Polyatomic Ions: When dealing with polyatomic ions (ions composed of multiple atoms), treat them as a single unit. If multiple polyatomic ions are needed, enclose the ion in parentheses and place the appropriate subscript outside. For example, in aluminum sulfate, the sulfate ion is enclosed in parentheses with a subscript of 3: (SO₄)₃.
- Transition Metals: Some metals can form cations with different charges. In such cases, Roman numerals indicate the ion’s positive charge. For example, iron(III) chloride is FeCl₃, where iron has a +3 charge.
- Simplest Ratio: The subscripts in the formula should represent the simplest whole-number ratio of ions that results in a neutral compound. For instance, magnesium oxide is MgO, indicating a 1:1 ratio of Mg²⁺ to O²⁻.
By following these guidelines, you can systematically determine the correct formulas for various ionic compounds.