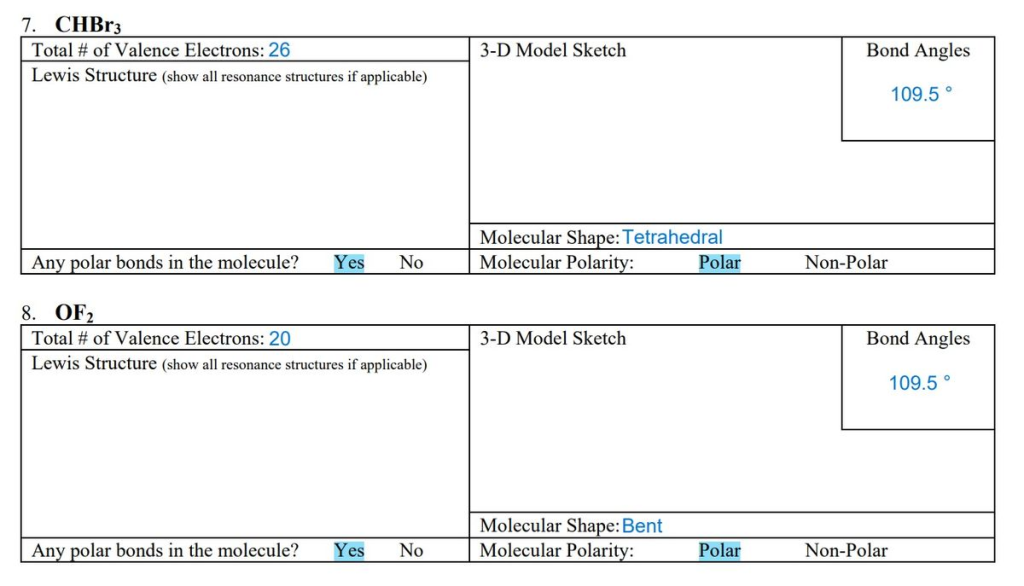
CHBr3 Total # of Valence Electrons: 26 Lewis Structure (show all resonance structures if applicable) Any polar bonds in the molecule? Yes No 8. OF2 Total # of Valence Electrons: 20 Lewis Structure (show all resonance structures if applicable) Any polar bonds in the molecule? Yes No 3-D Model Sketch Molecular Shape: Tetrahedral Molecular Polarity: 3-D Model Sketch Molecular Shape: Bent Molecular Polarity: Polar Polar Bond Angles Non-Polar 109.5° Bond Angles 109.5° Non-Polar
The Correct Answer and Explanation is :
Answers:
7. CHBr₃ (Bromoform)
- Total # of Valence Electrons: 26
- Carbon (C): 4 electrons
- Hydrogen (H): 1 electron
- Bromine (Br): 7 electrons × 3 = 21 electrons
- Total: 4 + 1 + 21 = 26 valence electrons
- Lewis Structure:
- Central carbon atom single-bonded to one hydrogen atom and three bromine atoms.
- Bromine atoms each have 6 lone electrons (3 lone pairs).
- No resonance structures exist because the bonds are fixed.
- Any Polar Bonds in the Molecule?: Yes
- C–Br bonds are polar because bromine is more electronegative than carbon.
- Molecular Shape: Tetrahedral.
- Molecular Polarity: Polar due to the asymmetrical distribution of electron density (bromine atoms create a dipole moment).
8. OF₂ (Oxygen Difluoride)
- Total # of Valence Electrons: 20
- Oxygen (O): 6 electrons
- Fluorine (F): 7 electrons × 2 = 14 electrons
- Total: 6 + 14 = 20 valence electrons
- Lewis Structure:
- Central oxygen atom single-bonded to two fluorine atoms.
- Oxygen has 4 lone electrons (2 lone pairs).
- Fluorine atoms each have 6 lone electrons (3 lone pairs).
- No resonance structures exist because the bonds are fixed.
- Any Polar Bonds in the Molecule?: Yes
- O–F bonds are polar because fluorine is more electronegative than oxygen.
- Molecular Shape: Bent.
- Molecular Polarity: Polar due to the asymmetrical shape and dipole moments.
- Bond Angles: Approximately 109.5°, as determined by the tetrahedral electron geometry.
Explanation:
The Lewis structures and polarity are determined by analyzing the total number of valence electrons and the bonding/geometry of the molecules:
- CHBr₃:
- Carbon forms four single bonds with hydrogen and bromine atoms, creating a tetrahedral shape.
- Bromine’s higher electronegativity makes the molecule polar because the dipoles don’t cancel out.
- OF₂:
- Oxygen, with two lone pairs and two bonds to fluorine, forms a bent molecular shape.
- Fluorine’s high electronegativity creates strong dipole moments, making the molecule polar.
Both molecules exhibit polar bonds, but their overall polarity arises from their 3D geometry.