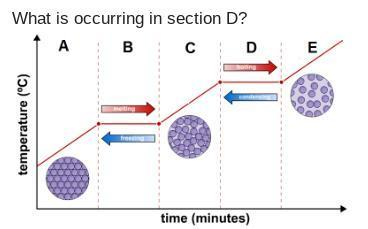
What is occurring in section D?
Heating curve phase changes
A. Particles of a gas are condensing into a solid.
B. Temperature of a gas is decreasing.
C. Temperature of a liquid is rising.
D. Particles of a gas are condensing into a liquid
The Correct answer and Explanation is:
In section D of a heating curve, the correct answer is D. Particles of a gas are condensing into a liquid.
Explanation
A heating curve represents how a substance changes phase as heat is added or removed. Each segment of the curve corresponds to different phases: solid, liquid, and gas, as well as the phase transitions between them, like melting, boiling, and condensation.
During section D, the system is transitioning from a gas to a liquid, known as condensation. In this phase, gas particles lose energy, which leads to a decrease in kinetic energy and movement. As the temperature of the gas decreases, the particles begin to come closer together. The intermolecular forces, which were previously negligible in the gaseous state, start to dominate as the particles lose energy.
This transition occurs at a constant temperature, known as the dew point. Here, the energy being released during condensation is used to overcome the intermolecular forces, allowing the gas to change into a liquid without a temperature change. This phase is represented on the heating curve as a horizontal line, indicating that while heat is being removed, the temperature remains constant until all the gas has condensed into a liquid.
Understanding this process is crucial in many real-world applications, such as in meteorology (e.g., the formation of clouds), refrigeration, and even distillation processes. The concept of phase changes is fundamental in chemistry and physics, as it illustrates how energy and temperature interact during the transition between different states of matter.
Thus, section D accurately describes the condensation of gas particles into a liquid, highlighting the interplay of energy loss and the resultant change in physical state.
