Which is the state of matter shown in this figure ?
A. Solid
B. Liquid
C. Gas
D. Plasma
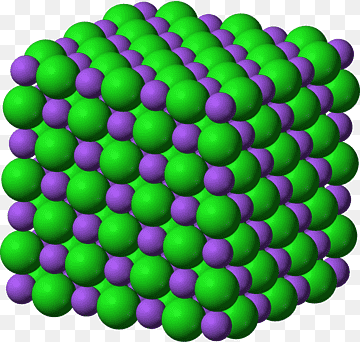
The Correct answer and Explanation is:
Based on the provided figure, the state of matter represented is A. Solid.
Explanation:
The image depicts sodium chloride (NaCl), commonly known as table salt, which is a classic example of an ionic compound. In solid-state matter, atoms or molecules are closely packed together in a fixed arrangement, resulting in a definite shape and volume. In the case of sodium chloride, it forms a crystalline structure characterized by a regular, repeating pattern of ions.
- Molecular Arrangement: In solids like NaCl, ions are held together by strong electrostatic forces known as ionic bonds. This bonding occurs between positively charged sodium ions (Na⁺) and negatively charged chloride ions (Cl⁻). The rigid structure of these ionic bonds means that the ions do not move freely, thus maintaining a fixed shape.
- Properties of Solids: Solids exhibit unique properties, such as incompressibility and rigidity. They have a specific volume and shape because the particles vibrate about fixed positions rather than moving freely as in liquids or gases.
- Comparison with Other States:
- Liquids have a definite volume but take the shape of their container. Molecules in liquids are less tightly packed than in solids, allowing them to slide past each other.
- Gases have neither a definite shape nor volume; gas molecules are much further apart and move freely.
- Plasma consists of highly charged particles with extremely high energy levels and is often found in stars and lightning.
In conclusion, the characteristics of sodium chloride, as shown in the image, firmly align with the properties of a solid. Thus, the correct answer is A. Solid.
